Question 12:
Diagram 11.1 shows two types of electrochemical cells, M and N.
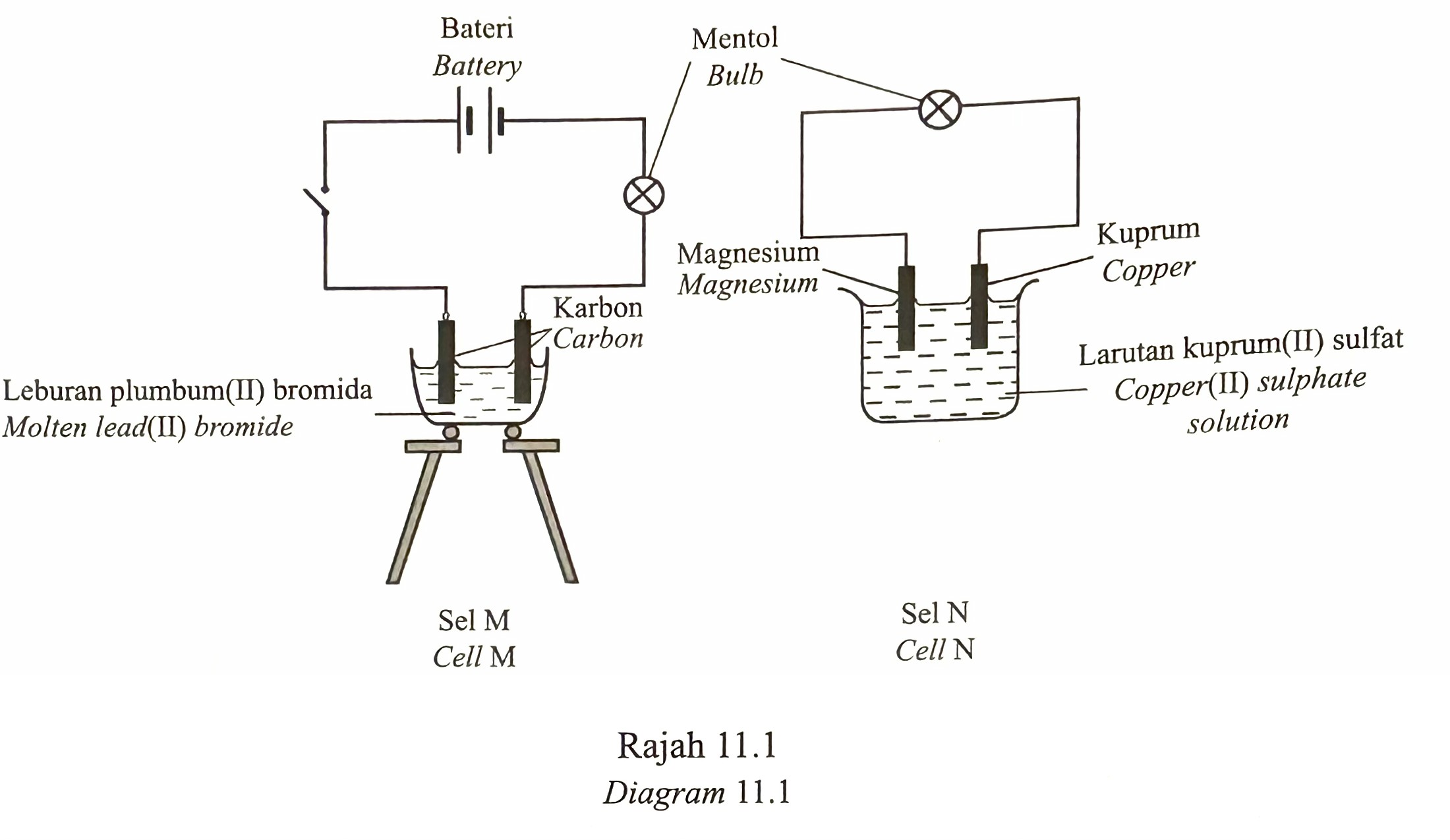
(a) State two types of electrochemical cell. [2 marks]
(b) State electrolytes for cells, M and N. [2 marks]
(c) State four differences between cells, M and N. [4 marks]
(d) Diagram 11.2 shows the apparatus set-up by a student to replace cell N.
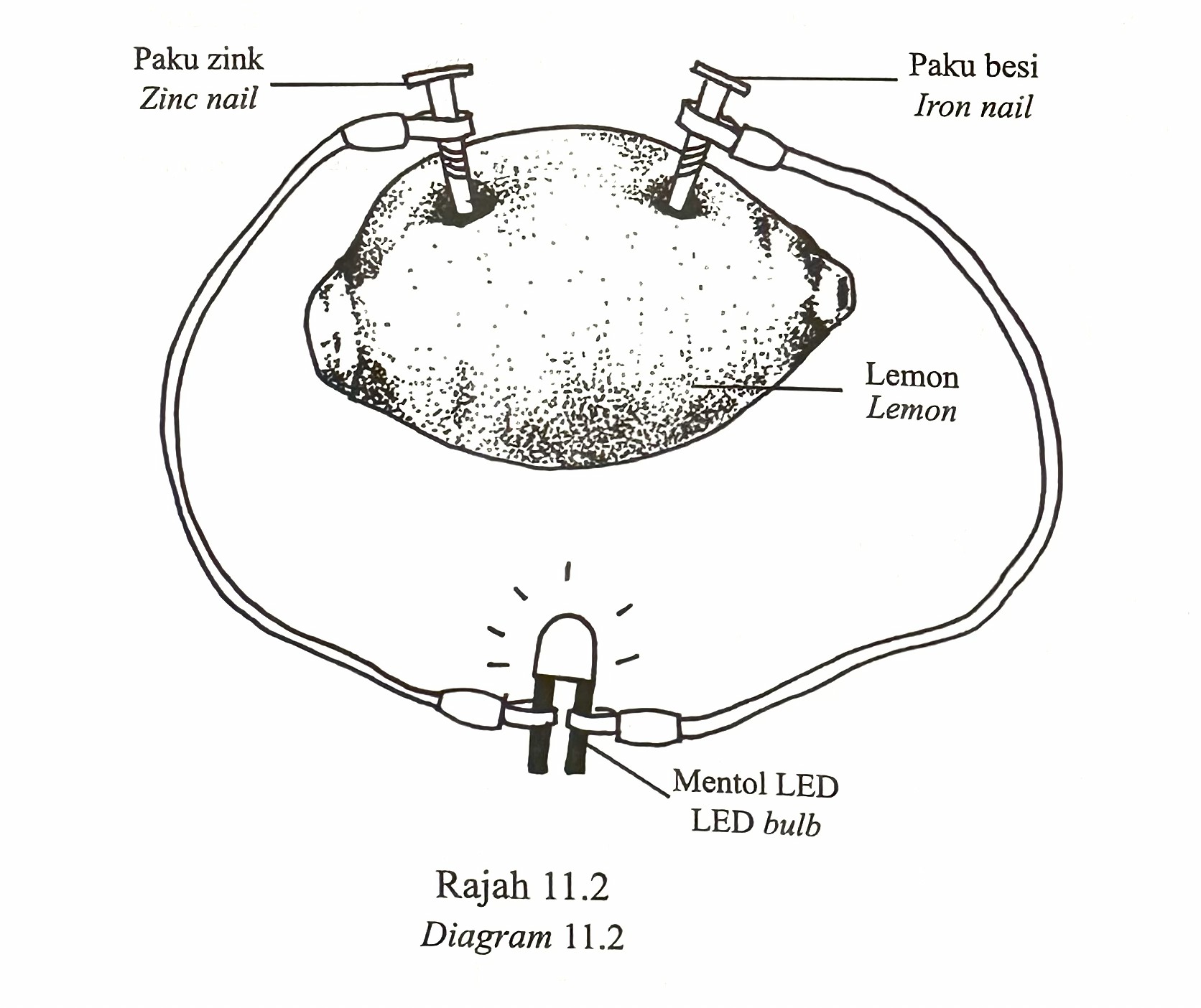 The student replaces the iron nail with a copper nail.
The student replaces the iron nail with a copper nail.
State the effect of the action in terms of the following aspects:
(i) The position of the metal pairs in the electrochemical series
(ii) Transfer of electrons
(iii) Voltage produced
(iv) Brightness of LED bulb
[4 marks]
Answer:
(a)
– Electrolytic cell
– Chemical cell
Explanation:
Electrochemical cells are divided into two main categories: chemical cells (generate electricity from spontaneous chemical reactions) and electrolytic cells (use electricity to induce nonspontaneous reactions).
(b)
Cell M : Molten lead(II) bromide
Cell N : Copper(II) sulphate solution
Explanation:
Electrolytes are substances that can conduct electric current when dissolved in water or in molten form, by producing ions. These ions carry electric current in solution. Determination of electrolytes in M and N cells based on the diagram.
(c)
– Cell M requires a battery as a source of electrical energy to decompose molten lead(II) bromide while the cell N does not require a battery because it produces its own electrical energy through the chemical reaction of the copper electrode and the magnesium electrode.
– Cells M use carbon (graphite) electrodes which do not react in electrolysis while cell N use metal electrodes, namely magnesium and copper, which react in the production of electrical energy.
– In the cell M , the anode is the positive terminal and the cathode is the negative terminal while in the cell N , the anode is the negative terminal and the cathode is the positive terminal.
– In the cell M , electrons flow from the positive terminal to the negative terminal while in the cell N , electrons flow from the negative terminal to the positive terminal.
Explanation:
Refer to the diagram and state the differences between electrolytic cells and chemical cells.
(d)(i)
The zinc-copper metal pair is farther apart in the electrochemical series compared to the zinc-iron pair.
Explanation:
The distance between zinc and copper in an electrochemical cell is greater than the distance between zinc and iron.
(d)(ii)
The transfer of electrons is greater because the difference in electropositivity between zinc and copper is greater than zinc and iron.
Explanation:
The difference in electropositivity between zinc and copper is larger than the difference between zinc and iron
(d)(iii)
The voltage produced by a zinc-copper electrode pair is higher than that of a zinc-iron electrode pair.
Explanation:
The difference in electropositivity between zinc and copper is larger than the difference between zinc and iron.
(d)(iv)
The LED bulb lights up brighter for the zinc-copper electrode pair because the current flowing is greater:
Explanation:
The difference in electropositivity between zinc and copper is larger than the difference between zinc and iron.
Diagram 11.1 shows two types of electrochemical cells, M and N.

(a) State two types of electrochemical cell. [2 marks]
(b) State electrolytes for cells, M and N. [2 marks]
(c) State four differences between cells, M and N. [4 marks]
(d) Diagram 11.2 shows the apparatus set-up by a student to replace cell N.
 The student replaces the iron nail with a copper nail.
The student replaces the iron nail with a copper nail.State the effect of the action in terms of the following aspects:
(i) The position of the metal pairs in the electrochemical series
(ii) Transfer of electrons
(iii) Voltage produced
(iv) Brightness of LED bulb
[4 marks]
Answer:
(a)
– Electrolytic cell
– Chemical cell
Explanation:
Electrochemical cells are divided into two main categories: chemical cells (generate electricity from spontaneous chemical reactions) and electrolytic cells (use electricity to induce nonspontaneous reactions).
(b)
Cell M : Molten lead(II) bromide
Cell N : Copper(II) sulphate solution
Explanation:
Electrolytes are substances that can conduct electric current when dissolved in water or in molten form, by producing ions. These ions carry electric current in solution. Determination of electrolytes in M and N cells based on the diagram.
(c)
– Cell M requires a battery as a source of electrical energy to decompose molten lead(II) bromide while the cell N does not require a battery because it produces its own electrical energy through the chemical reaction of the copper electrode and the magnesium electrode.
– Cells M use carbon (graphite) electrodes which do not react in electrolysis while cell N use metal electrodes, namely magnesium and copper, which react in the production of electrical energy.
– In the cell M , the anode is the positive terminal and the cathode is the negative terminal while in the cell N , the anode is the negative terminal and the cathode is the positive terminal.
– In the cell M , electrons flow from the positive terminal to the negative terminal while in the cell N , electrons flow from the negative terminal to the positive terminal.
Explanation:
Refer to the diagram and state the differences between electrolytic cells and chemical cells.
(d)(i)
The zinc-copper metal pair is farther apart in the electrochemical series compared to the zinc-iron pair.
Explanation:
The distance between zinc and copper in an electrochemical cell is greater than the distance between zinc and iron.
(d)(ii)
The transfer of electrons is greater because the difference in electropositivity between zinc and copper is greater than zinc and iron.
Explanation:
The difference in electropositivity between zinc and copper is larger than the difference between zinc and iron
(d)(iii)
The voltage produced by a zinc-copper electrode pair is higher than that of a zinc-iron electrode pair.
Explanation:
The difference in electropositivity between zinc and copper is larger than the difference between zinc and iron.
(d)(iv)
The LED bulb lights up brighter for the zinc-copper electrode pair because the current flowing is greater:
Explanation:
The difference in electropositivity between zinc and copper is larger than the difference between zinc and iron.