Question 1:
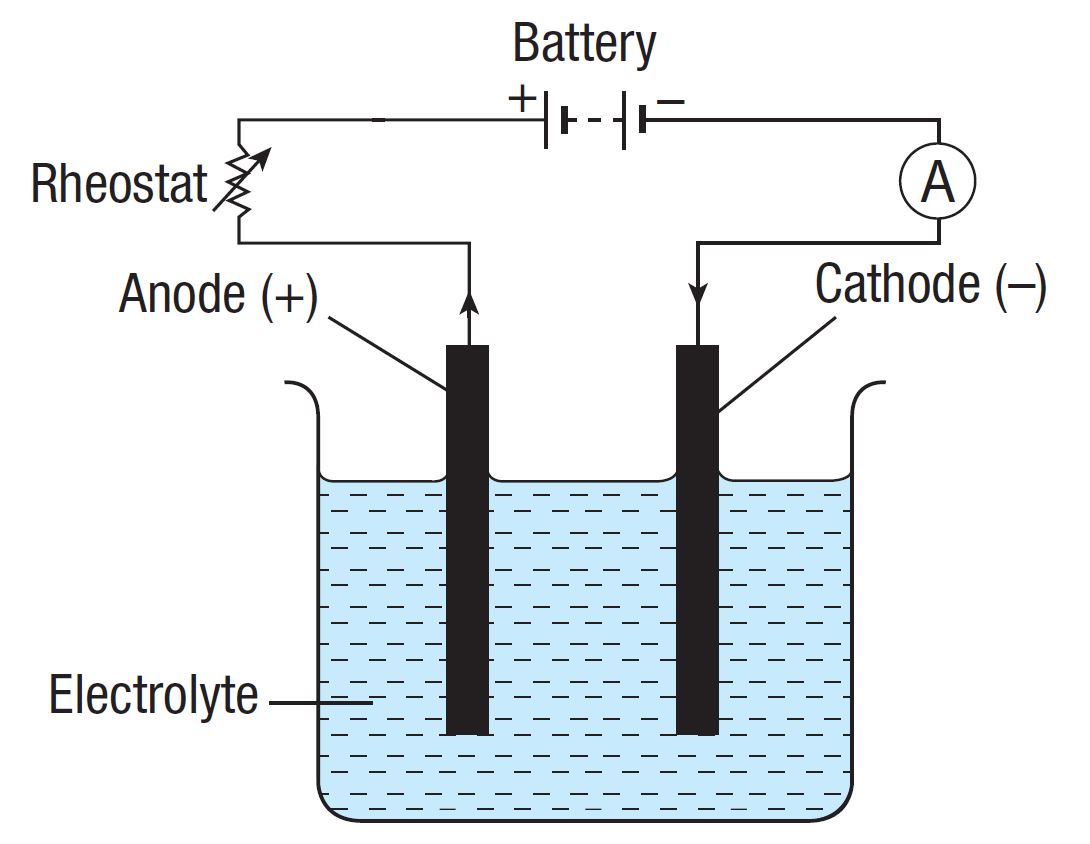
Draw and label the structures of an electrolytic cell.
Draw and label the structures of an electrolytic cell.
Answer:
Question 2:
Describe the movement of ions to electrodes during electrolysis.
Describe the movement of ions to electrodes during electrolysis.
Answer:
During electrolysis, positive ions (cations) move to the cathode (negative electrode) and negative ions (anions) move to the anode (positive electrode).
Question 3:
Give four examples of applications of electrolysis in industries.
Give four examples of applications of electrolysis in industries.
Answer:
Extraction of metal, purification of metal, electroplating of metal and wastewater treatment using electrocoagulation