Question 1:
Figure 1.1 shows the formation of bronze.
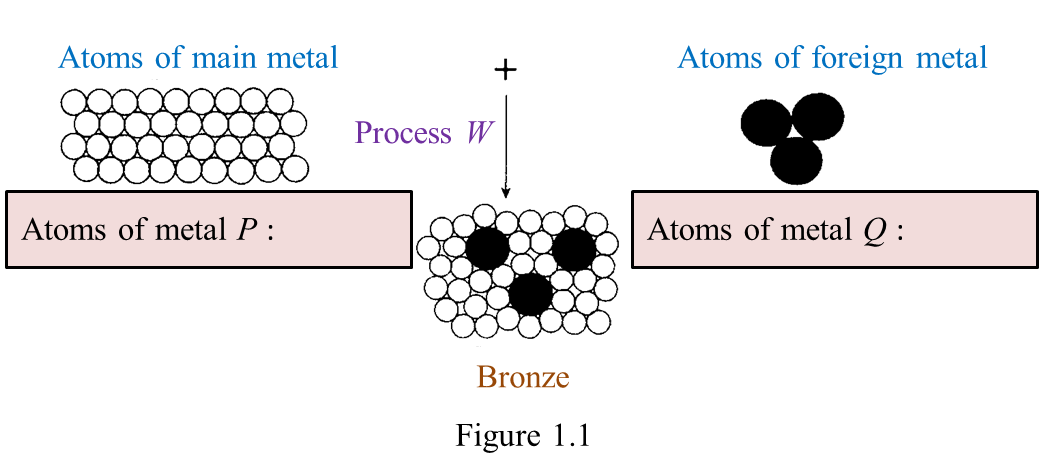
(a) On figure 1.1, name the atoms of metals, P and Q. [2 marks]
(b) Name process W. [1 mark]
(c)(i) State one property of bronze. [1 mark]
(ii) State the effect of the atoms of metal Q in bronze. [1 mark]
 (d) The medal in Figure 1.2 is made of bronze.
(d) The medal in Figure 1.2 is made of bronze.
Give one property of the medal if it is made of metal P only. [1 mark]
Answer:
(a)
P: Copper
Q: Tin
(b)
Alloying
(c)(i) Harder/ can withstand corrosion better than copper.
(c)(ii) The atoms of metal Q in bronze prevent the layers of atoms of metal W in the bronze from sliding easily over one another.
(d) Softer, more malleable, less resistant to corrosion.
Figure 1.1 shows the formation of bronze.

(a) On figure 1.1, name the atoms of metals, P and Q. [2 marks]
(b) Name process W. [1 mark]
(c)(i) State one property of bronze. [1 mark]
(ii) State the effect of the atoms of metal Q in bronze. [1 mark]
 (d) The medal in Figure 1.2 is made of bronze.
(d) The medal in Figure 1.2 is made of bronze.Give one property of the medal if it is made of metal P only. [1 mark]
Answer:
(a)
P: Copper
Q: Tin
(b)
Alloying
(c)(i) Harder/ can withstand corrosion better than copper.
(c)(ii) The atoms of metal Q in bronze prevent the layers of atoms of metal W in the bronze from sliding easily over one another.
(d) Softer, more malleable, less resistant to corrosion.
Question 2:
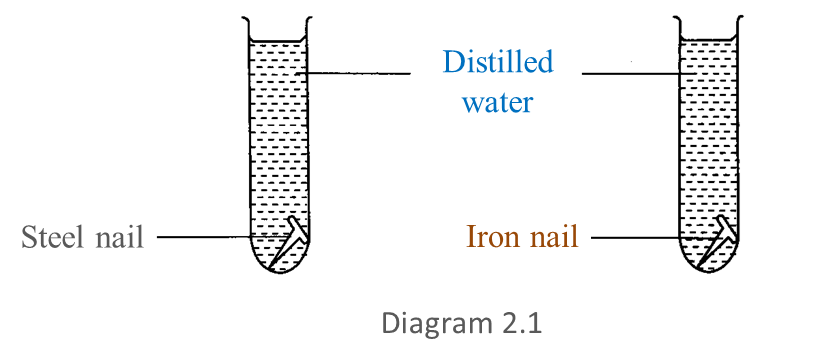
Diagram 2.1 shows an experiment to study the resistance of steel alloy towards corrosion.
After 3 days, the result obtained is shown in Diagram 2.2.
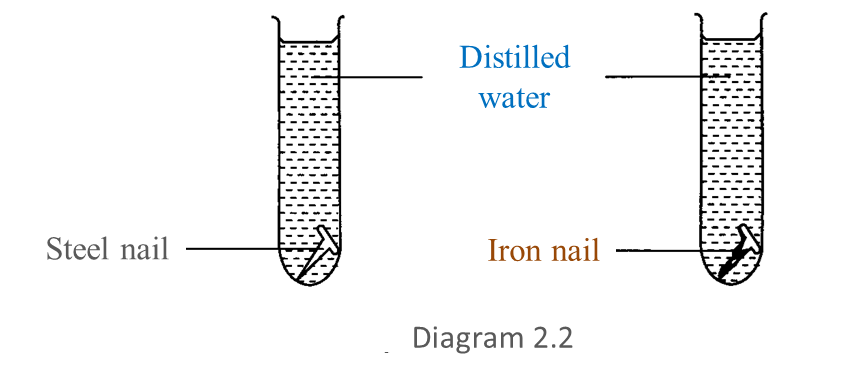
(a) Based on Diagram 2.2, write down one observation for this experiment. [1 mark]
(b) Write down one hypothesis for this experiment. [1 mark]
(c) State the variables in this experiment.
(i) Constant variable [1 mark]
(ii) Manipulated variable [1 mark]
(d) Predict the condition of the steel nail on the 5th day. [1 mark]
(e) Based on this experiment, state the operational definition for an alloy. [1 mark]
Answer:
(a) The iron nail has corroded while the steel nail has not.
(b) Steel is more resistant to corrosion than iron.
(c)(i) Distilled water, number of days the nails are immersed in water.
(any one)
(c)(ii) Type of metal (steel on iron)
(d) The steel nail will remain not corroded
(e) An alloy is a mixture of metal which is harder and more resistant to corrosion than a pure metal.
Diagram 2.1 shows an experiment to study the resistance of steel alloy towards corrosion.

After 3 days, the result obtained is shown in Diagram 2.2.

(a) Based on Diagram 2.2, write down one observation for this experiment. [1 mark]
(b) Write down one hypothesis for this experiment. [1 mark]
(c) State the variables in this experiment.
(i) Constant variable [1 mark]
(ii) Manipulated variable [1 mark]
(d) Predict the condition of the steel nail on the 5th day. [1 mark]
(e) Based on this experiment, state the operational definition for an alloy. [1 mark]
Answer:
(a) The iron nail has corroded while the steel nail has not.
(b) Steel is more resistant to corrosion than iron.
(c)(i) Distilled water, number of days the nails are immersed in water.
(any one)
(c)(ii) Type of metal (steel on iron)
(d) The steel nail will remain not corroded
(e) An alloy is a mixture of metal which is harder and more resistant to corrosion than a pure metal.