Question 1:
Diagram below shows the extraction of tin ore at high temperature in a blast furnace.
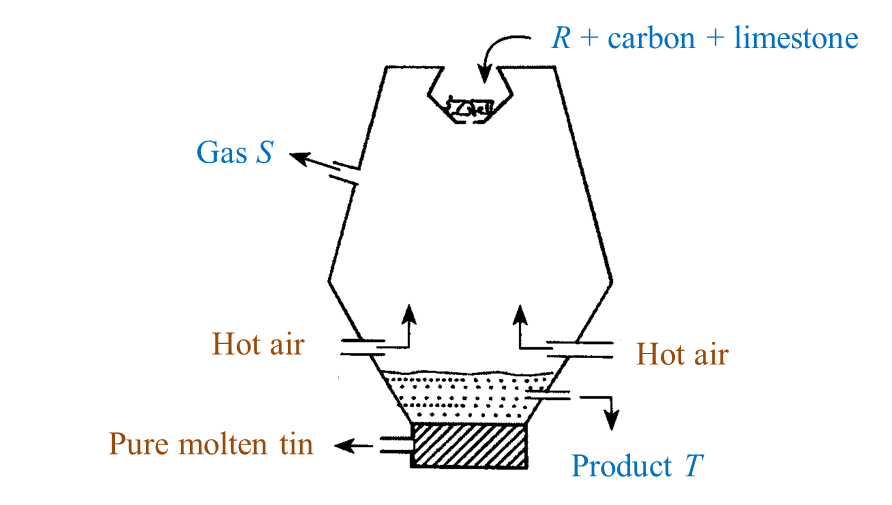
(a) Name two elements in R. [2 marks]
(b) State one reason why carbon is a suitable element to use in the extraction of tin ore from R? [1 mark]
(c) Name gas S. [1 mark]
(d) What is the function of limestone in this process? [1 mark]
(e) Name product T. [1 mark]
Answer:
(a) Tin, oxygen
(b) As tin is less reactive than carbon, carbon can be used to reduce the tin ore from R.
(c) Carbon dioxide.
(d) The limestone is decomposed to produce quicklime (calcium oxide) which combines with impurities such as silica to form slag.
(e) Slag
Diagram below shows the extraction of tin ore at high temperature in a blast furnace.

(a) Name two elements in R. [2 marks]
(b) State one reason why carbon is a suitable element to use in the extraction of tin ore from R? [1 mark]
(c) Name gas S. [1 mark]
(d) What is the function of limestone in this process? [1 mark]
(e) Name product T. [1 mark]
Answer:
(a) Tin, oxygen
(b) As tin is less reactive than carbon, carbon can be used to reduce the tin ore from R.
(c) Carbon dioxide.
(d) The limestone is decomposed to produce quicklime (calcium oxide) which combines with impurities such as silica to form slag.
(e) Slag