05d Application of Reactivity Series of Metals
Quiz-summary
0 of 5 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
Information
SPM Form 4 Science Chapter 5
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading...
You must sign in or sign up to start the quiz.
You have to finish following quiz, to start this quiz:
Results
0 of 5 questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 points, (0)
Categories
- Not categorized 0%
- 1
- 2
- 3
- 4
- 5
- Answered
- Review
-
Question 1 of 5
1. Question
1 pointsCoke is used to extract certain metals from their ore. Which of the following metals can be extracted from their ore by heating with coke?
I. Aluminium
II. Zinc
III. Lead
IV. IronCorrect
Well done! You are correct.
Incorrect
The correct answer is “II, III and IV Only”.
-
Question 2 of 5
2. Question
1 pointsMagnesium can’t be extracted from its ore by heating with carbon because
Correct
Well done! You are correct.
Incorrect
The correct answer is “Magnesium is more reactive than carbon “.
-
Question 3 of 5
3. Question
1 points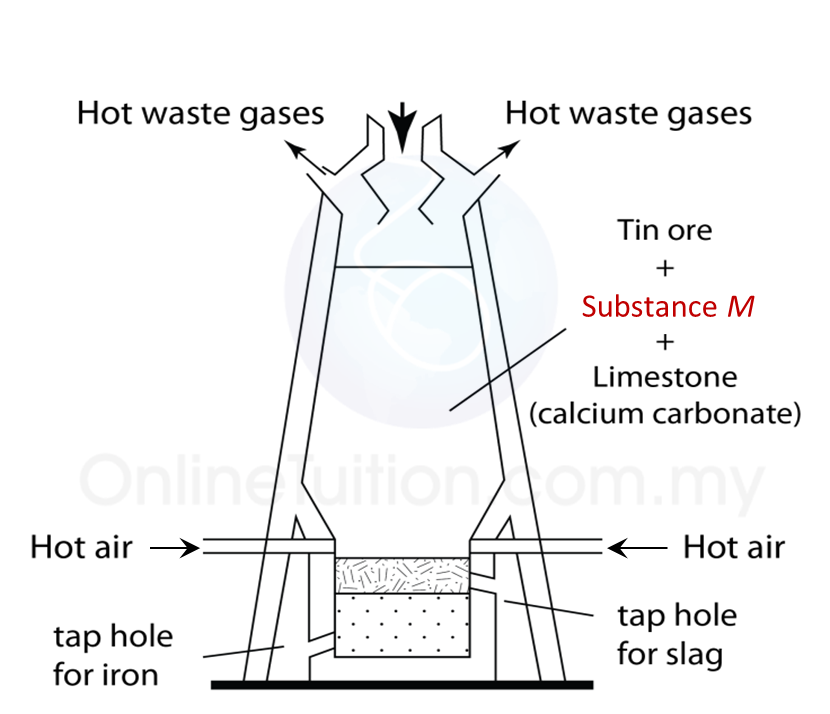
The figure shows a blast furnace used to extract a metal in industry. What is substance M?
Correct
Well done! You are correct.
Incorrect
The correct answer is “Carbon”.
-
Question 4 of 5
4. Question
1 pointsTin oxide + Carbon → Tin + Carbon dioxide
Equation above represents the reaction in the process of extracting tin from tin ore.
What is the purpose of adding limestone in the process?Correct
Well done! You are correct.
Incorrect
The correct answer is “To react with the impurities to form slag “.
-
Question 5 of 5
5. Question
1 pointsWhich metal can be extracted from its ore through the process of reduction using carbon?
Correct
Well done! You are correct.
Incorrect
The correct answer is “Tin”.