Table 1 shows several elements with their proton numbers and nucleon numbers respectively.
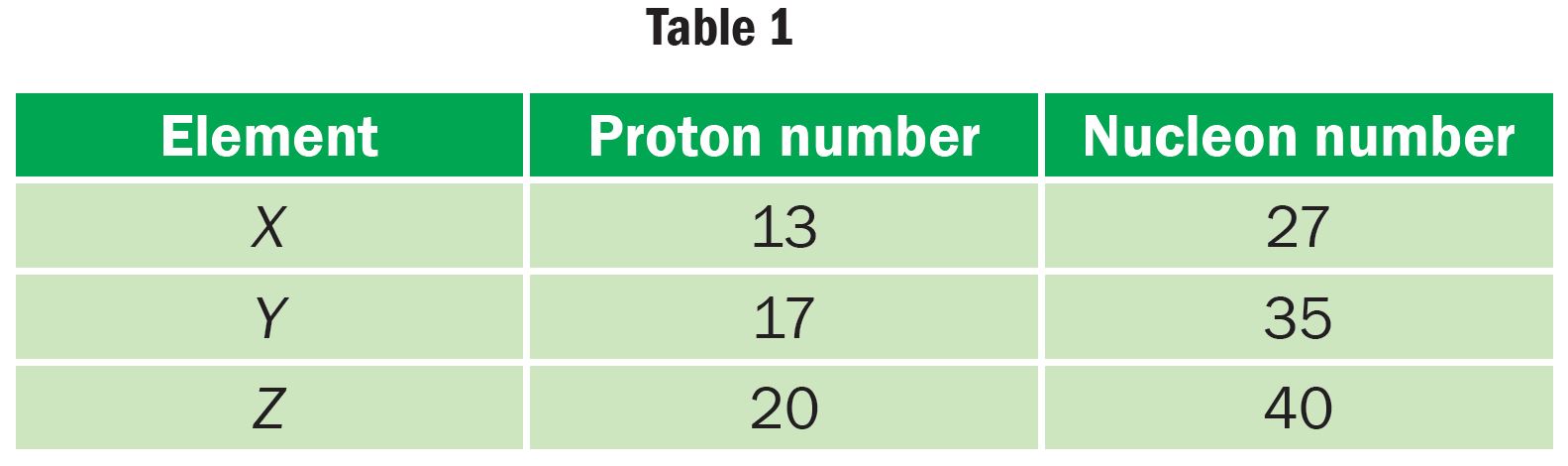
(a) Determine the number of neutrons for the atomic elements X, Y and Z.
(b) Draw the electron arrangement for the atomic element X, Y and Z.
(c) In your opinion, where are the elements X, Y and Z located in the Modern Periodic Table of Elements?
(d) How do atoms of the following elements achieve a stable electron arrangement?
(i) Element X
(ii) Element Y
Answer:
(a)
X: 14
Y: 18
Z: 20
(b)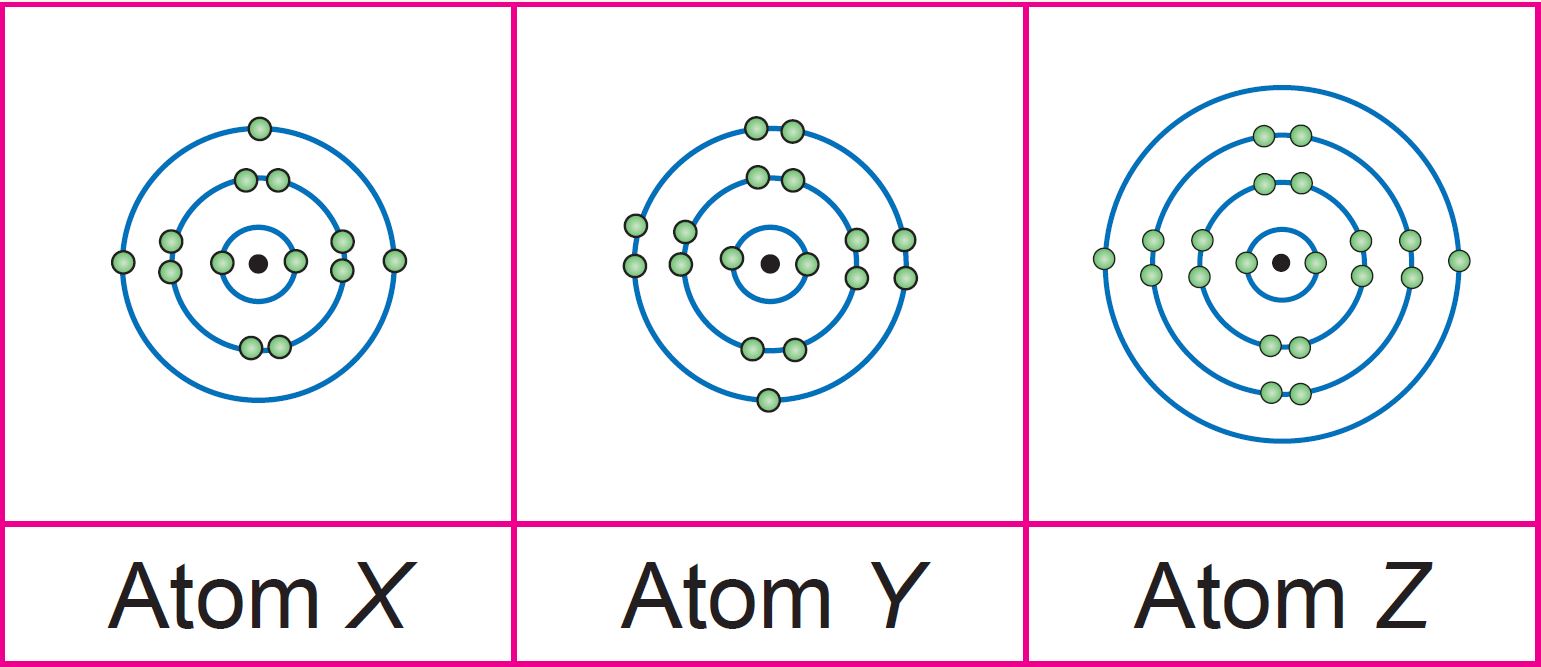
(c)
X: Group 13, Period 3
Y: Group 17, Period 3
Z: Group 2, Period 4
(d)(i)
Atom X will donate three electrons to the atom of another element to form a positive ion.
(d)(ii)
Atom Y will accept one electron from the atom of another element to form a negative ion.
Table 2 shows the elements P, Q, R and S with their respective proton and nucleon numbers.
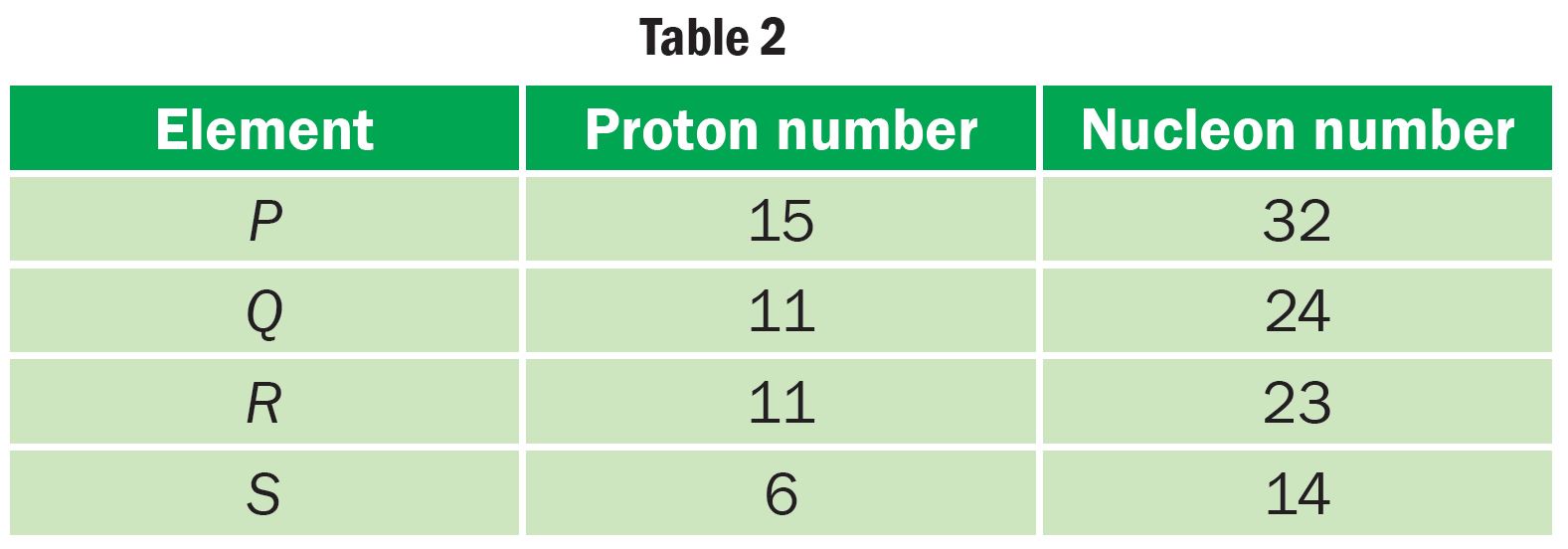
(a) Which element is a pair of isotope? Explain your answer.
(b) State the uses of the isotopes you mentioned in question 4(a) in medicine and in the field of industry.
(c) State element P and its uses in agriculture.
(d) Name element S and state its uses in agriculture and archaeology.
Answer:
(a)
Q and R, because atom Q and R have the same proton number but different nucleon number.
(b)
Industry: is used to detect leakage in underground pipes
Medical: detect clogged blood vessels
(c)
Phosphorus-32: is used to detect the rate of absorption of phosphorus fertiliser in plants
(d)
Carbon-14
Agriculture: to detect the rate of photosynthesis in plants
Archaeology: to determine the age of fossils and artifacts