Question 1:
Give two examples of alloy containing copper metal.
Give two examples of alloy containing copper metal.
Answer:
Bronze / Brass / Duralumin
Question 2:
Draw the arrangement of atoms in an alloy and a pure metal, then relate the arrangement of the atoms to the properties of pure metal.
Draw the arrangement of atoms in an alloy and a pure metal, then relate the arrangement of the atoms to the properties of pure metal.
Answer:
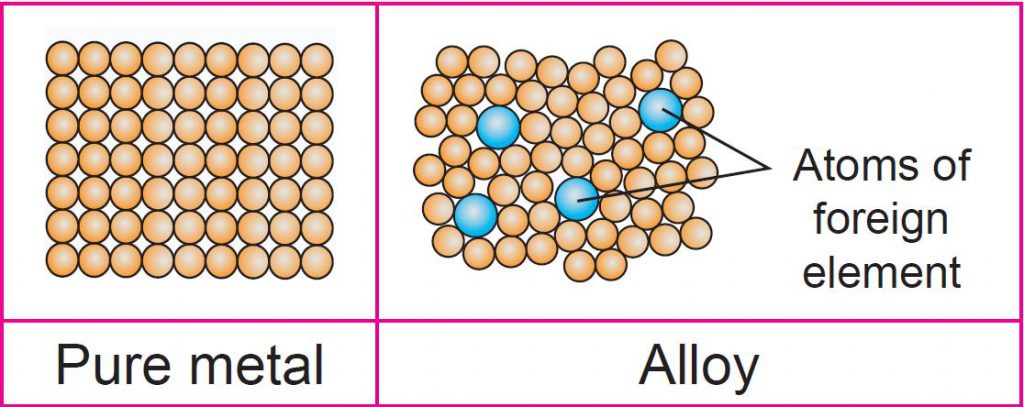
• Pure metal: atoms in the pure metal are arranged in an orderly manner and in layers. The layer of atoms in pure metal easily slides over each other when a force is applied.
• Alloy: The difference in size of foreign atom disrupts the orderly arrangement of metals. The layer of atoms in alloy is difficult to slide over each other when a force is applied. Therefore, alloy is harder.
Question 3:
What alloy is used to make the body of an aeroplane? Explain why the alloy is chosen.
What alloy is used to make the body of an aeroplane? Explain why the alloy is chosen.
Answer:
Duralumin.
Duralumin is strong, light and resistant to corrosion.