Electrolysis
1. Electrolysis is a process where a compound is separated into its constituent elements when electric current passes through an electrolyte.
2. In electrolysis, energy is changed as shown below:
3. The apparatus used in an electrolytic cell consists of a dry cell or battery, an electrolyte and two electrodes as shown below.
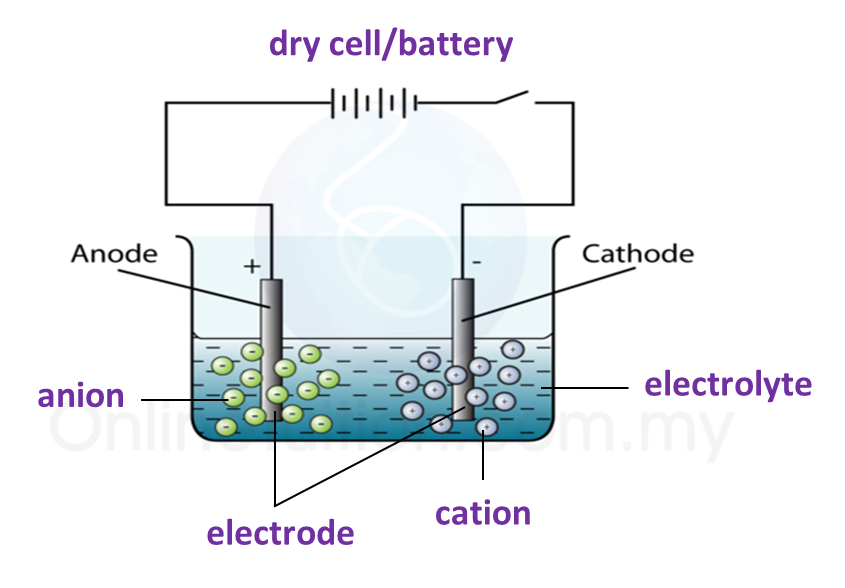

Electrolytic cell
Electrolyte
(a) An electrolyte is a compound in a molten form or in aqueous solution which conducts electric current.
(b) Electrolyte contains two types of charged ions which move freely:
(i) Ion with positive charge (cation), for example, metal ions and hydrogen ions.
(ii) Ion with negative charge (anion), for example, non-metal ions.
(i) Ion with positive charge (cation), for example, metal ions and hydrogen ions.
(ii) Ion with negative charge (anion), for example, non-metal ions.
(c) Example of electrolyte: molten potassium chloride and hydrocloric acid.
Electrode
(a) Electrode is a conductor which is immersed in an electrolyte and connected to an electric source.
(b) Examples of electrode: carbon (graphite) and platinum.
(c) The electrode connected to the positive terminal of the cell is positive electrode and is given a name, anode.
(d) The electrode connected to the negative terminal of the cell is negative electrode and is called the cathode.
Ammeter
Ammeter is used to detect the flow of current in the circuit.
Dry cell or battery
The source that generates electrical energy.