Figure 1 shows an apparatus set-up to study the electrolysis of an aqueous copper(II) sulphate solution, CuSO4 using different electrodes as shown in electrolytic cell P and electrolytic cell Q.
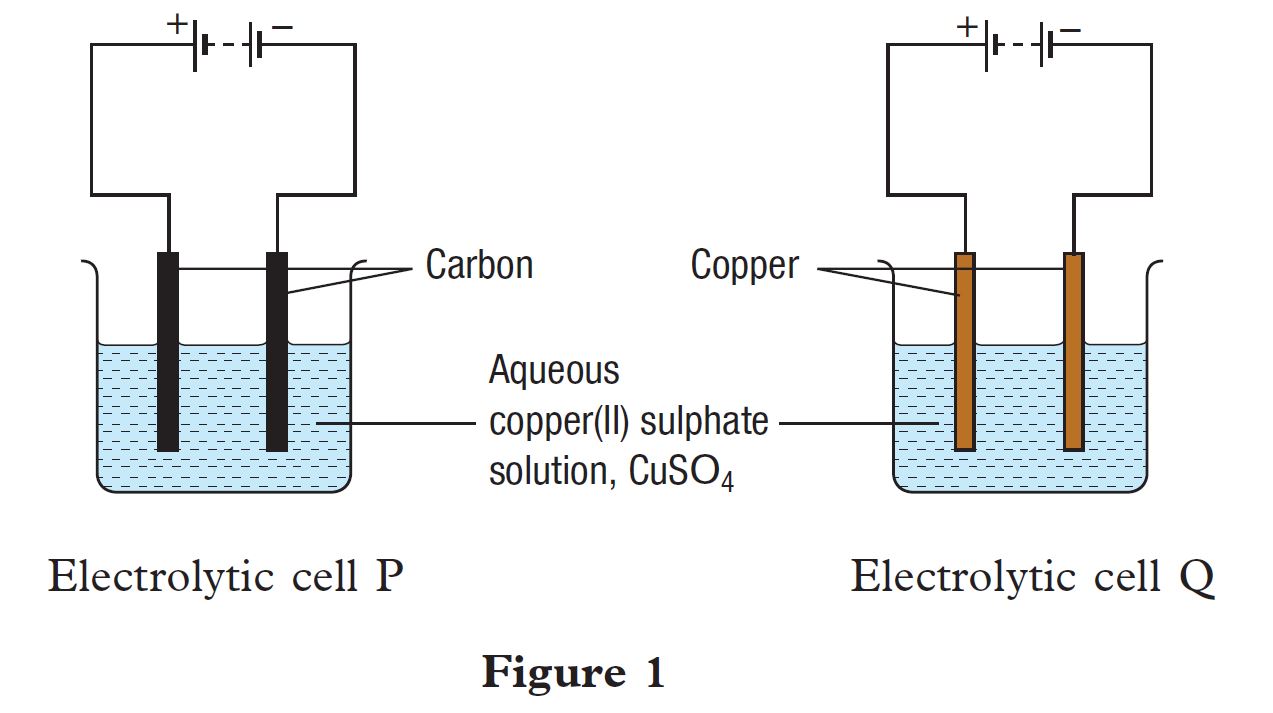
(a) State the meaning of electrolysis.
(b) State all the ions present in the aqueous copper(II) sulphate solution.
(c) Name the ions discharged at the anode and cathode for the following electrolytic cells:
(i) electrolytic cell P
at anode:
at cathode:
(ii) electrolytic cell Q
at anode:
at cathode:
(d) Name one example of the application of electrolysis in industries which applies the electrolysis concept of electrolytic cell Q.
Answer:
(a)
Electrolysis is the process of decomposition of a compound in the molten or aqueous state into its constituent elements when electric current flows through it.
(b)
Copper(II) ion, Cu2+, hydrogen ion, H+, sulphate ion, SO4 2–, hydroxide ion, OH–
(c)(i)
At anode: Hydroxide ion
At cathode: Copper(II) ion
(c)(ii)
At anode: No ion is discharged
At cathode: Copper(II) ion
(d)
Purification of metal