Photograph 1 shows two types of substances.

(a) State the type of particles that form:
(i) salt
(ii) sugar
(b) Give two other examples of substances that are formed from the same type of particles as salt and sugar.
(c) Give two examples of substances that contain the oxygen that exists in the form of molecules and ions.
Answer:
(a)(i)
Salt: ions
(a)(ii)
Sugar: molecules
(b)
Ions: marbles / limewater / acid / alkali
Molecules: carbon dioxide / oxygen / nitrogen / naphthalene / alcohol
(c)
Molecules: oxygen gas/carbon dioxide gas/sulphur dioxide gas Ions: zinc oxide/iron oxide
Figure 1 shows part of the Modern Periodic Table of Elements.
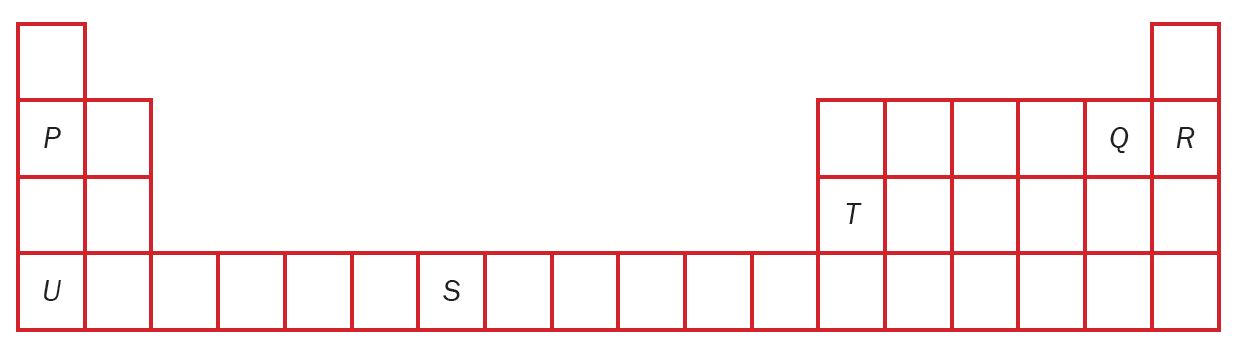
P, Q, R, S, T and U are the letters used to represent the elements in the Modern Periodic Table of Elements which is not the correct symbol of that element.
(a) How are these elements arranged in the Modern Periodic Table of Elements?
(b) Which elements belong to the same group?
(c) Which element exists as a stable atom? Explain your answer.
(d) An element has a nucleon number of 32 and a proton number of 16. Where is the element located in the Modern Periodic Table of Elements?
(e) State the electron arrangement of the following elements:
(i) P
(ii) Q
(iii) T
Answer:
(a)
The elements are arranged from left to right and top to bottom, in the order of their increasing proton numbers.
(b)
P and U
(c)
Element R.
Element R has achieved a stable octet electron arrangement.
(d)
Group 16, Period 3
(e)(i)
P: 2.1
(e)(ii)
Q: 2.7
(e)(iii)
T: 2.8.3