Question 1:
Give one example of a fast reaction and one example of a slow reaction in daily life.
Give one example of a fast reaction and one example of a slow reaction in daily life.
Answer:
Example of a fast reaction: Burning of candle
Example of a slow reaction: Photosynthesis
(any other suitable answer is accepted)
Question 2:
Define rate of reaction.
Define rate of reaction.
Answer:
Rate of reaction is the change in the quantity of reactants or products of a reaction per unit time.
Question 3:
Figure 1 shows the graph of volume of hydrogen gas released against time.
Answer:
(a)
Average rate of reaction for the first 2 minutes
Figure 1 shows the graph of volume of hydrogen gas released against time.
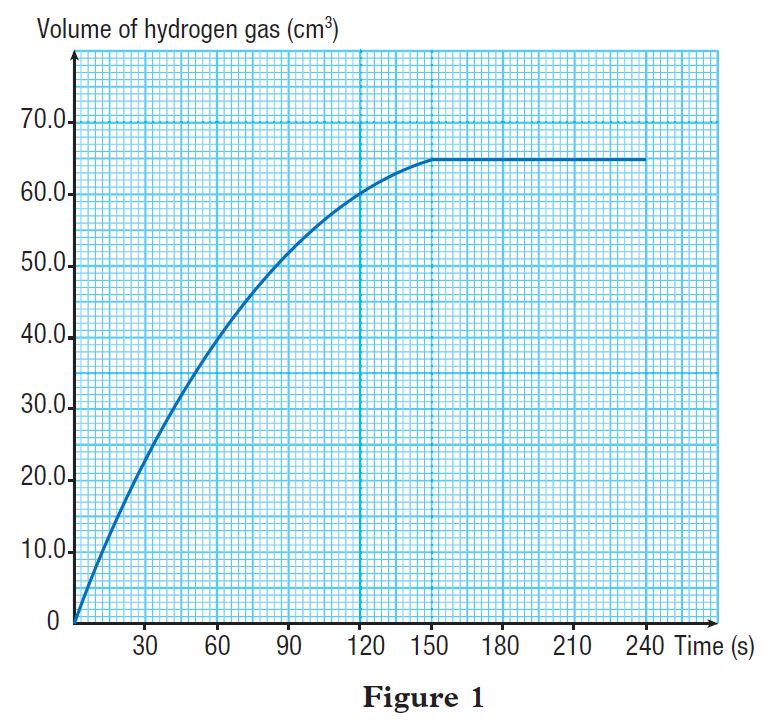
Calculate the average rate of reaction:
(a) for the first 2 minutes
(b) in the second minute
(c) for the whole reaction
Answer:
(a)
Average rate of reaction for the first 2 minutes
(b)
Average rate of reaction in the second minute
(c)
Average rate of reaction for the whole reaction