Electrolysis of Copper (II) Chloride Solution
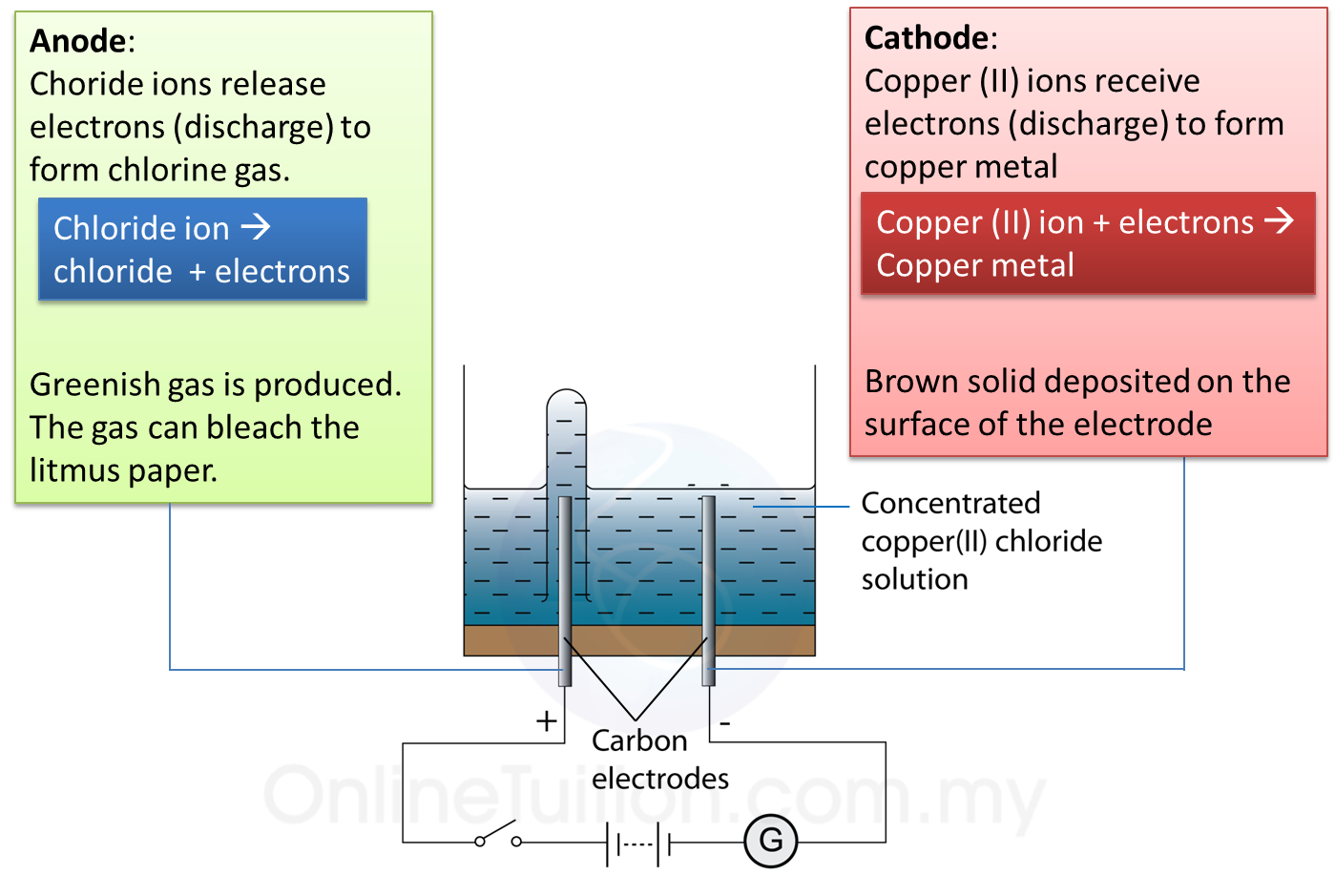
1. Electrolysis of copper (II) chloride solution.
(a) Copper (II) ion with positive charge will attract to cathode to discharge as a copper.
(b) Chloride ion will attract to anode to discharge as a chlorine gas.
(c) At anode, chloride ions lose electrons. Greenish gas which can bleach the litmus paper is produced.
(d) At cathode, copper (II) ion receives electron. Brown solid deposited on the surface of the electrode.

2. Thus, electrolysis of copper (II) chloride produces copper and chlorine gas.