5.4.2 Reactivity Series and Extraction of Metals
1. The method that is used in the extraction of metal from its are depends on the position of the metal in the reactivity series of metals.
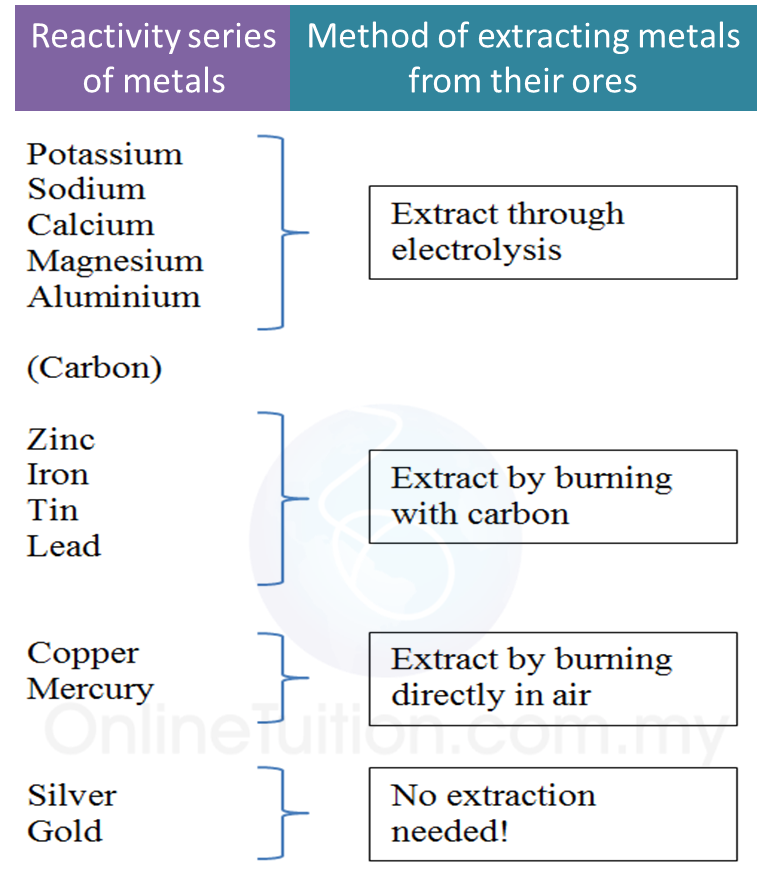
2. Metals which are located higher than carbon in the reactivity series are extracted from their molten ores using the electrolysis method.
3. Metals which are located lower than carbon in the reactivity series are extracted using the reduction method with coke (or carbon).
4. Carbon is used in the extraction process because
(a) It is cheap
(b) Easily obtained
5. Metals located the lowest in the reactivity series like silver and gold can be extracted naturally without any complex chemical reaction. These metals exist as free elements in the Earth’s crust.