4.4 Classification of Elements in the Period Table
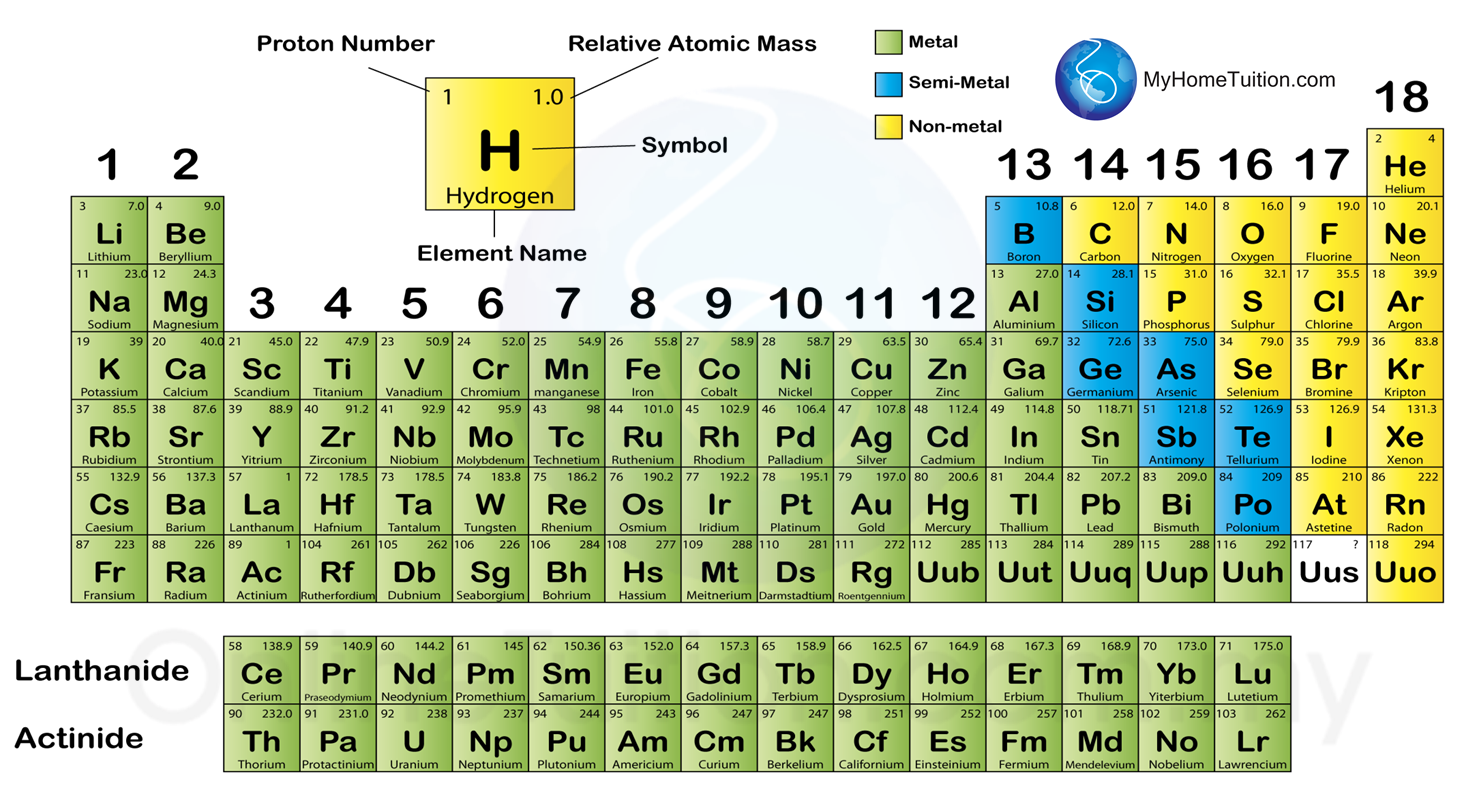
4.4.1 The classification of Elements
1. In modern periodic table, the elements are arranged in ascending order of proton number.
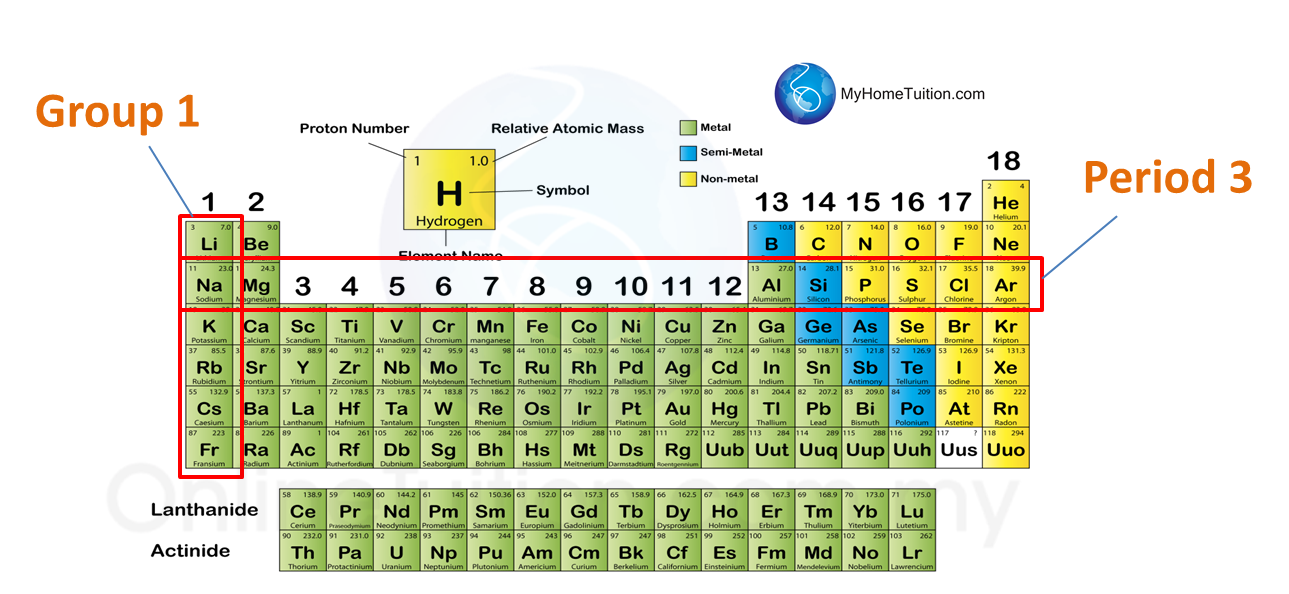
Group
1. The vertical columns of the periodic table is called group.
2. There are 18 groups in the periodic table.
3. Elements in the same group have similar chemical properties.
Example:
Elements in Group 1 react violently with water to produce metal hydroxide, hydrogen and heat.
4. Elements in groups 1 and 2 are very reactive metals (except hydrogen).
5. Elements between groups 2 and 13 are called the transition elements.
6. Transition elements are metallic elements. Most of them are hard and shiny. All transition elements are good conductors of electricity.
7. The elements in group 17 are non-metals called halogens.
8. Group 18 consists of noble (inert) gases, which are inactive gases.
Period
1. The horizontal rows is called the period.
2. There are 7 periods in period table.
3. The first period has 2 elements only.
4. The second and third period consist of 8 elements, are called the short period.
5. The forth and the fifth period consist of 18 elements, are called the long period.
6. The sixth and the seventh period have 32 elements.
7. The chemical and physical properties of the elements are gradually changed when crossing periods.
4.4.2 Metals, Semi-metals and Non-metals
1. The elements in the Periodic Table are divided into metals, semi-metals and non-metals.
2. Most elements in group 1, 2, 13 and transition elements are metals.
3. Most non-metal elements are located in groups 16, 17 and 18.
4. Seven elements between metals and non-metals are semi-metals. Semi-metals possess certain properties of metals and non-metals.
5. When moving across a period from left to right, the properties of metal change gradually to those of semi-metal and finally non-metal.
6. The metals become increasingly active as they move down the table.
4.4.3 Importance of the Periodic Table
1. The periodic table enables us to study the elements in an orderly and systematic way.
2. It helps us to know the properties of elements that fall into a particular group.
3. It enables us to predict the properties, reactions and uses of the elements.