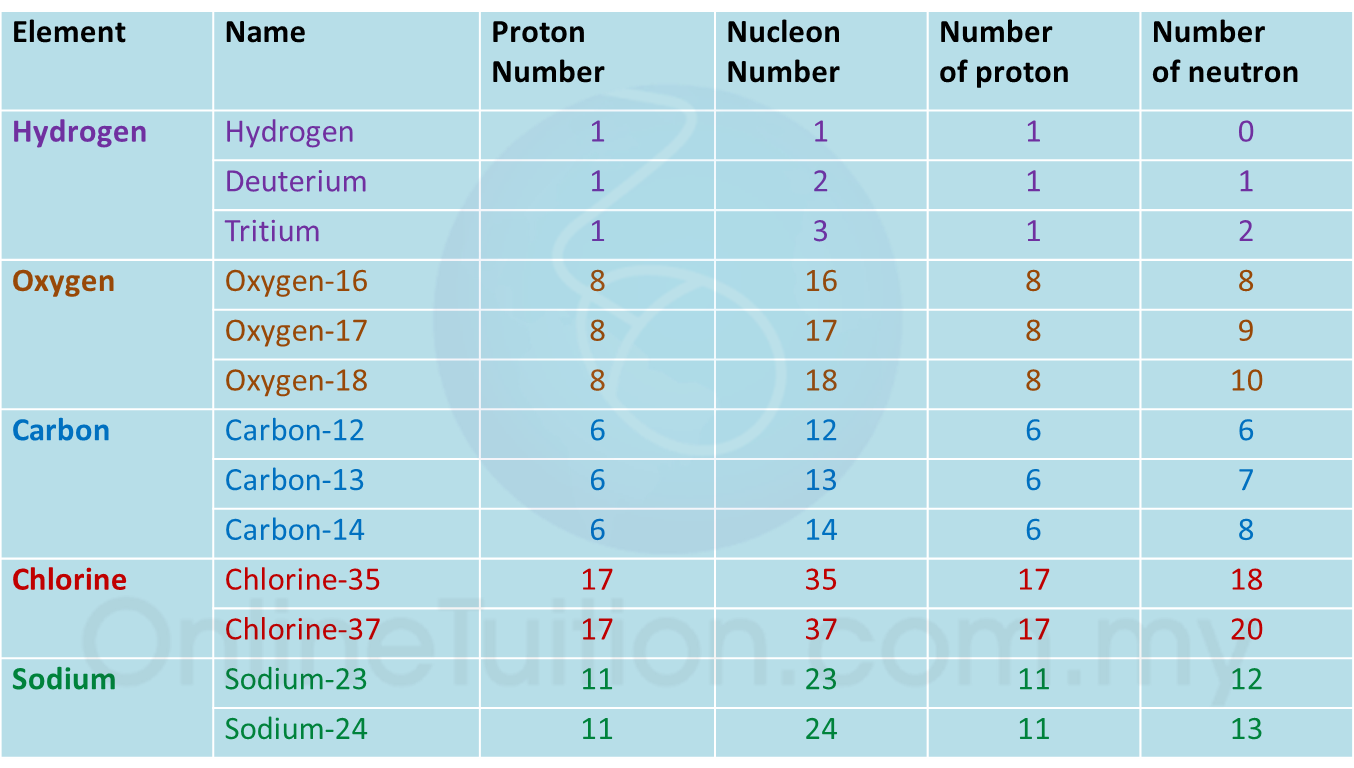
4.3.2 Isotopes
1. Isotopes are atoms of certain elements which have the same number of protons but different number of neutrons in the nucleus of the atoms.
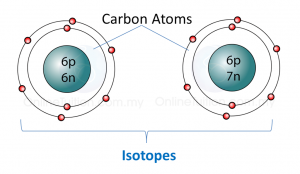
2. Isotopes of the same element have same chemical properties. For example, both carbon-12 and carbon-13 burn in oxygen to form carbon dioxide.
3. Physical properties of isotopes are different. For example, carbon-14 isotope has a melting point, boiling point and density that are higher than those of carbon-12 and carbon-13.
Examples of isotopes